Veritor SARS-CoV-2 & Flu A+B Assays, 30 tests/kit (Short-Dated, Minimum Expiry Lead is 60 days; Non-returnable; Non-refundable) (Continental US Only)
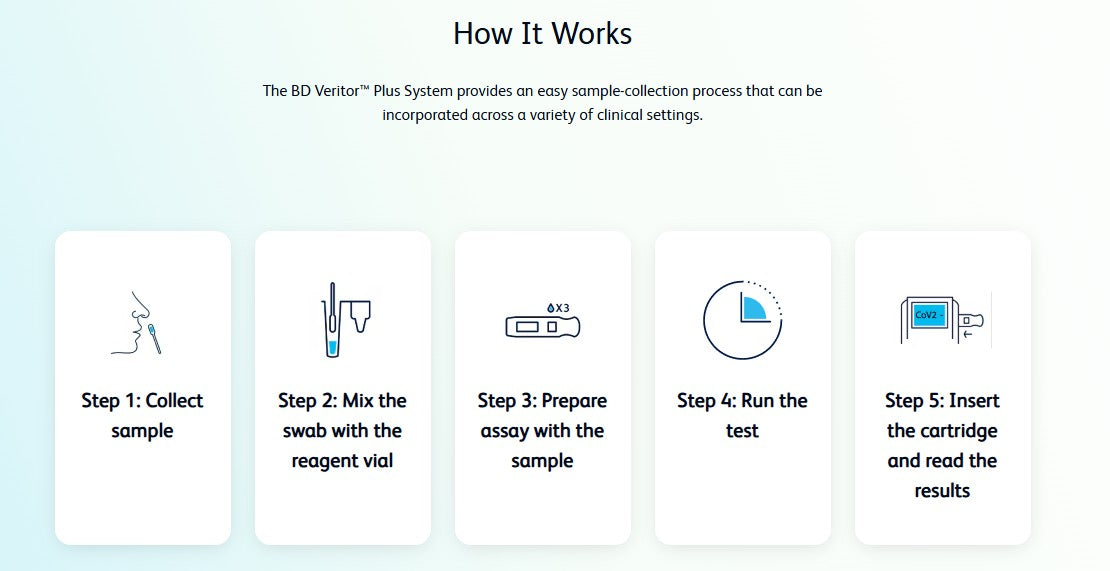
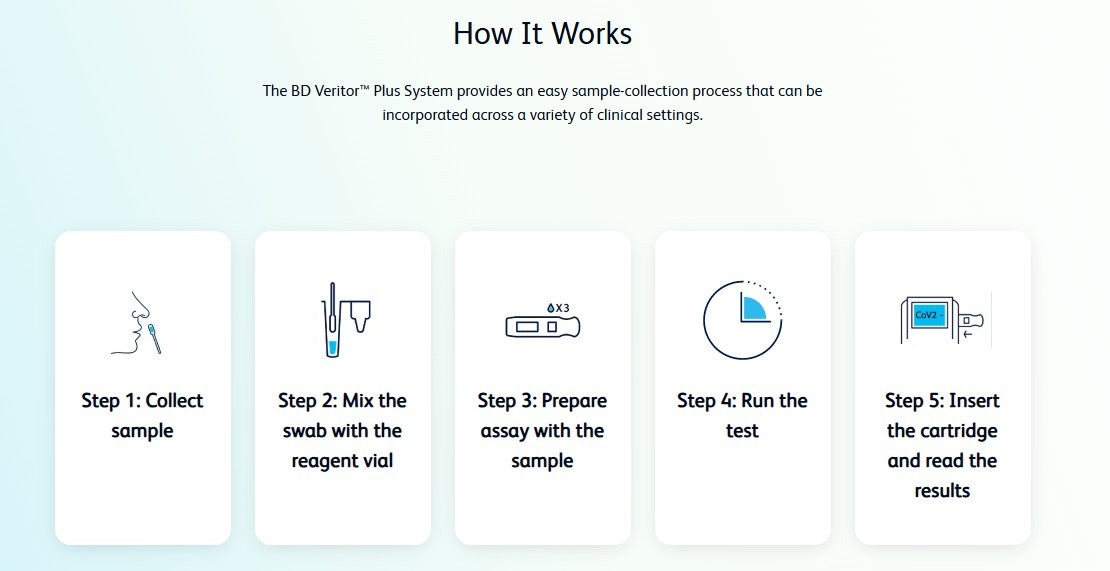
Test for COVID-19, Flu A and Flu B* with a single sample, using the 3-in-1 assay from the BD Veritor Plus System.
The BD Veritor Plus System for Rapid Detection of SARS-CoV-2 & Flu A+B* provides results for 3 different respiratory viruses (SARS-CoV-2 & Flu A, and Flu B*) from one nasal specimen. With multiple testing modes and workflow efficiencies, the Analyzer is more versatile than ever.
|
|
PPA |
NPA |
Results in |
|
SARS-CoV-2 |
86.7% (95% CI, 75.8%-93.1%) |
99.5% (95% CI, 97.4%-99.9%) |
15 minutes
|
|
Influenza A |
82.7% (95% CI, 74.9%-88.5%) |
97.5% (95% CI, 95.7%-98.5%) |
15 minutes |
|
Influenza B |
80.7% (95% CI, 70.3%-88.1%) |
98.2% (95% CI, 95.7%-99.3%) |
15 minutes |
SARS-CoV-2, Flu A and Flu B* results in 15 minutes
NPA=negative percent agreement; PPA=positive percent agreement.
|
Ordering information |
Cat no. |
Qty |
|
BD Veritor™ Plus System SARS-CoV-2 & Flu A+B kit* |
256088 |
30 tests |
|
BD Veritor™ Plus Analyzer |
1 unit |
Get more answers with every sample.
Simplifies the testing process
May help reduce manual test processing errors with its easy operation and single-button functionality
Provides results traceability
Captures or downloads the lot number, patient/ specimen ID, operator ID, and test records using the BD Veritor™ InfoWiFi module Provides result traceability
Delivers workflow efficiency
Adapts easily to your workflow by offering 2 operational modes
- Walk Away: the test device is inserted immediately into the Analyzer so staff can multitask while the sample incubates (15 minutes)
- Analyze Now: the test device is inserted after the incubation time is complete, allowing batches of samples to be tested (results in seconds)
Achieves reliable, rapid results
Displays easy-to-read digital results for COVID-19 and Flu A+B* in 15 minutes1 :
- “CoV2: +” for positive; “CoV2: –” for negative
- “FLU A: +” for positive; “FLU A: –” for negative
- “FLU B: +” for positive; “FLU B: –” for negative
Provides clear, objective results by correcting for nonspecific binding and detecting faint lines not recognized by the unaided eye1
----------------------------------------------------------------------------------------------
A negative result does not rule out COVID-19 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. A negative result should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management. For additional information, please refer to the HCP fact sheet.
1. BD Veritor™ Plus System for Rapid Detection of SARS-CoV-2 and Flu A+B. Package insert. 500051910 Becton, Dickinson and Company.
BD Life Sciences, 7 Loveton Circle, Sparks, MD 21152-0999 USA 800-638-8663
*EUA Information for the BD Veritor™ SARS-CoV-2 and SARS-CoV-2 & Flu A+B assays: These products have not been FDA cleared or approved; but have been authorized by FDA under EUA for use by authorized laboratories. The BD Veritor™ System for Rapid Detection of SARS-CoV-2 has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; the BD Veritor™ System for Rapid Detection of SARS-CoV-2 & Flu A+B has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens; and, the emergency use of these products is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.