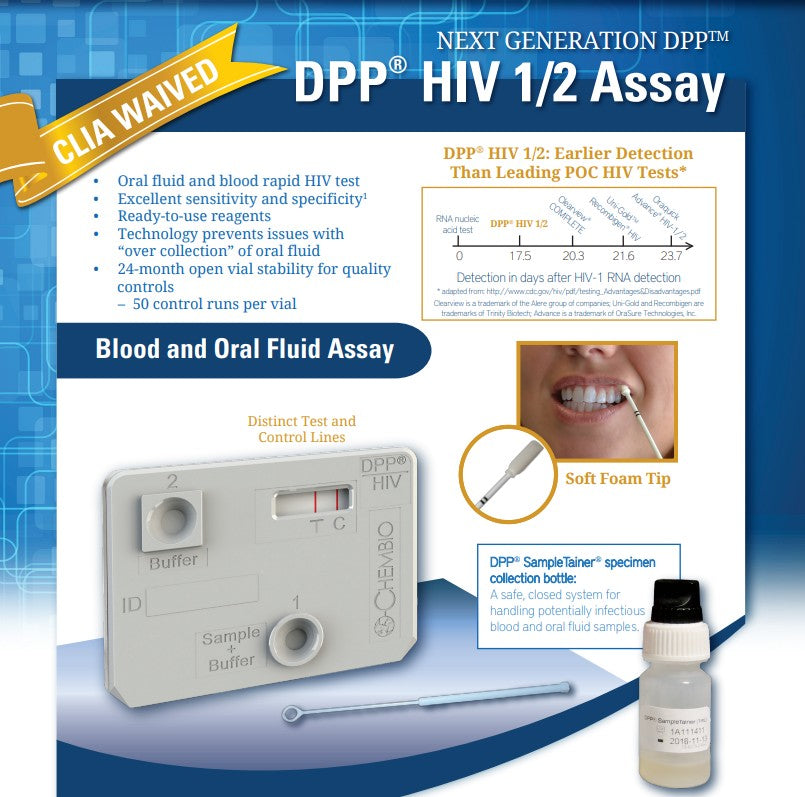
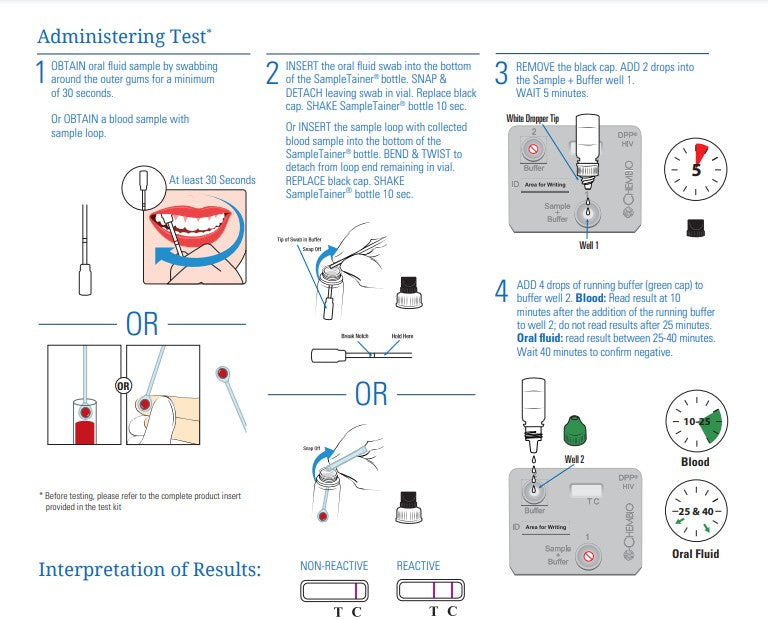
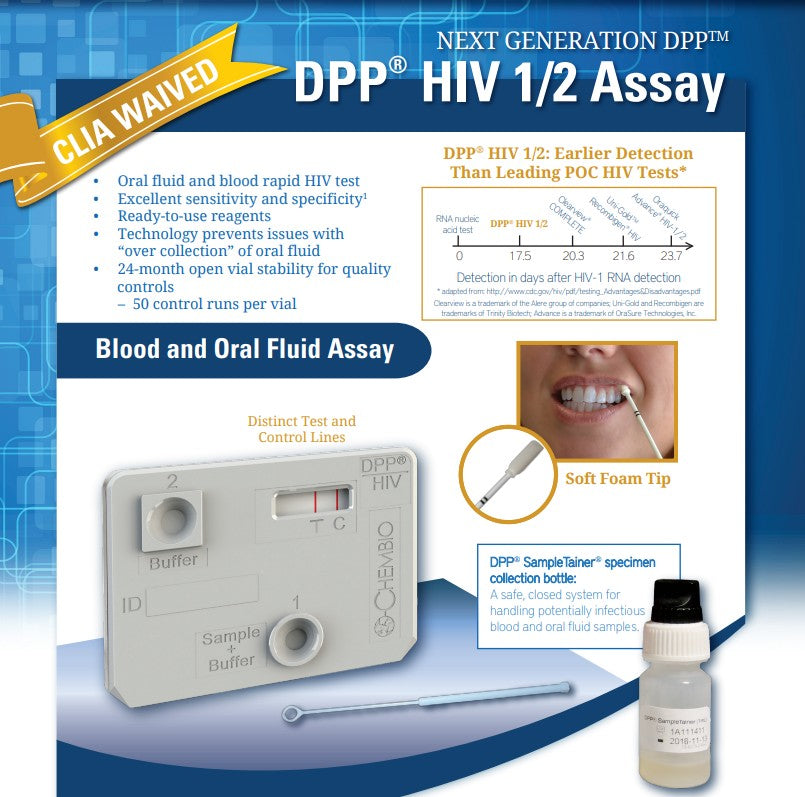
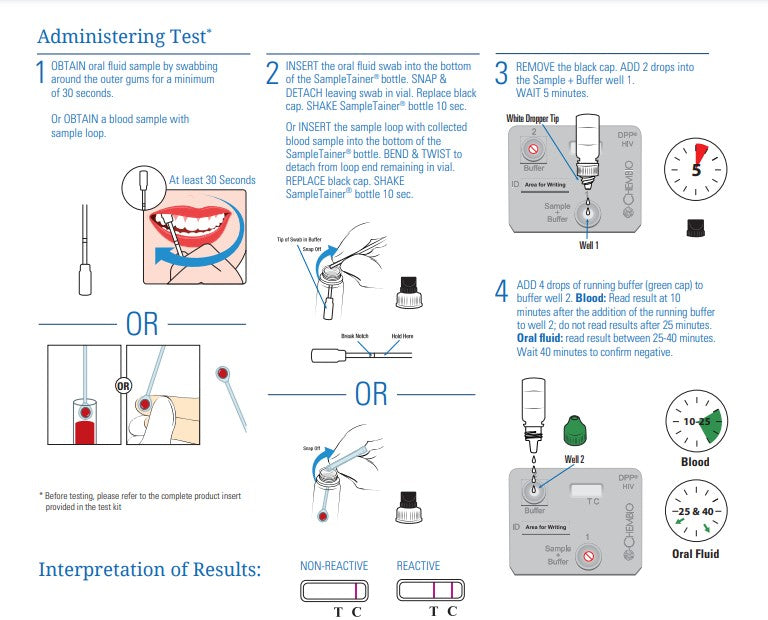
An FDA approved, rapid point-of-care test that detects antibodies to HIV-1 and HIV-2
Contents:
(20) Individually Pouched DPP HIV 1/2 Test Devices with desiccant pouch
(1) Quick Reference Instructions (for CLIA Waived)
(20) Copies of Subject Information Notice
(20) Oral Fluid Swabs
(20) Disposable 10μL Sample Loops
(20) DPP HIV SampleTainer Bottle – BLACK Cap
(1) DPP HIV Running Buffer – GREEN Cap
(1) Product Insert for the DPP HIV 1/2 Assay
Flexible
- Works with oral fluid, fingerstick blood, venous whole blood, serum and plasma
Convenient
- Low sample volume: only 10 μl
- Long Shelf life: 23 months
- Safe, closed system for sample handling with DPP SampleTainer
Accurate