In the reverse testing algorithm for syphilis, you will need to reflex to RPR to find out if you have an active or past infection. For HIV you would need to confirm a positive based on your site’s protocols.
DPP HIV-Syphilis Promo Pack, 400 tests (20 kits; 20 tests/kit) (CDI 100-0102-1)
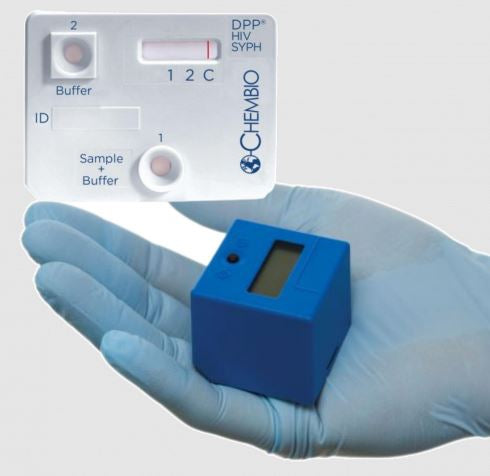
An FDA Approved, CLIA Waived dual rapid test for the detection of antibodies to HIV 1/2 and Treponema pallidum in fingerstick whole blood, venous whole blood, or plasma specimens.
Reliable
- Patented DPP technology allows for higher sensitivity: >99% for HIV and >94% for T. pallidum
- Built-in procedural control
Easy to Use
- Small sample volume: 10 μl
- 15 minute dual rapid test
- Provides objective results using simple, handheld digital reader (DPP Micro Reader)
Economical
- Combined reimbursement: CPT Codes 86703, 86780
- Ideal as the first line rapid test in your algorithm
| Manufacturer # | 65-9502-0 |
| Brand | DPP |
| Manufacturer | Chembio Diagnostic |
| Country of Origin | USA |
| Application | Sexual Health Test Kit |
| CLIA Classification | CLIA Waived |
| Contents |
20 Kits + Micro Reader Each Kit Includes: |
| Number of Tests | 20 Tests/Kit, 20 Kits/Box (400 Tests) |
| For Use with | For use with DPP Micro Reader |
| Sample Type | Whole Blood Sample |
| Specialty | Immunoassay |
| Test Format | Cassette Format |
| Test Kit Type | Rapid Test |
| Test Name | HIV-Syphilis |
| Test Type | Antibody Test |
| Time to Results | 15 Minute Results |
| Product | Catalog Number |
| DPP HIV-Syphilis (20 tests) FDA Approved | 65-9502-0 |
| DPP HIV-Syphilis Rapid Test Control Pack | 65-9555-0 |
| DPP Micro Reader for Use with DPP HIV-Syphilis | 70-1056-0 |
FAQ:
Could temperature be a factor when using this test in the field or on the street?
The test components should be at room temperature, 18 to 25 C (64 to 77 F), before performing the test and the test should be performed within the same temperature range. If the temperature of the testing area falls outside of 18 to 25 C (64 to 77 F) it is recommended to perform QC prior to testing.
Is it possible to visually interpret the test?
No. The test must be read using the DPP Micro Reader.
Is there any data regarding efficacy with pregnant patients?
Yes, we Chembio has multiple sets of data in our IFU that addresses the test performance in this population of patients.
Is this test 4th generation for HIV?
We do not detect antigen to HIV, just antibodies to HIV, the following papers support this approach to the rapid HIV Testing.
The paper ‘Field Accuracy of fourth Generation Rapid Test’ by Lewis et al analyzed 4 studies that included 17,381 samples screened using 4th generation (Antigen-Antibody) rapid tests.
Findings:
- 26 reference-standard-defined acute HIV infections were missed
- 35 false positives were observed
Lesson’s Learned: ‘Fourth-generation RDTs are currently unsuitable for the detection of acute HIV-1’ at the point of care.
Why the Poor Test Performance: The assay detects free HIV-1 p24 antigen. This has a short window of detection. “Antibodies form immune complexes with antigen in the bloodstream and preclude binding of antigen to the test strip. This technical difficulty can be overcome with an antigen dissociation step, but this may be hard to implement at the point of care.”
Is the concern for syphilis biological false positive the same as with traditional testing?
The term “biological false positive” for syphilis is defined as a positive reaction to regain tests (non-trep), but negative reaction to treponemal tests. Non-trep such as RPR, are looking for anti-cardiolipin antibodies which are not specifically associated with treponemal infections, so having a positive RPR and negative Treponemal test is what is usually referred to as a biological false positive when the traditional algorithm is used.
What is the shelf-life?
24 months from date of manufacture