The Flowflex Plus COVID-19 and Flu A/B Home Test is a lateral flow immunoassay intended for the qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B protein antigens. This test is authorized for non-prescription home use with self-collected anterior nares nasal swab specimens from individuals aged 14 years or older, or with adult-collected anterior nasal swab specimens from individuals two (2) years or older. This test is only authorized for individuals with signs and symptoms of respiratory infection consistent with COVID-19 within the first six (6) days of symptom onset when tested at least twice over three days with at least 48 hours between tests. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. The Flowflex Plus COVID-19 and Flu A/B Home Test does not differentiate between SARS-CoV and SARS-CoV-2 viruses and is not intended to detect influenza C antigens. All negative SARS-CoV-2 results are presumptive.
Clinical Performance:
The Flowflex Plus COVID-19 and Flu A/B Home Test was compared to FDA EUA and 510(k) RT-PCR COVID-19 and Flu A/B assays. The Flowflex Plus COVID-19 and Flu A/B Home Test correctly identified 90.6% of positive specimens and 99.3% of negative specimens for COVID-19, 91.7% of positive specimens and 99.8% of negative specimens for Flu A, and 93.6% of positive specimens and 99.7% of negative specimens for Flu B.
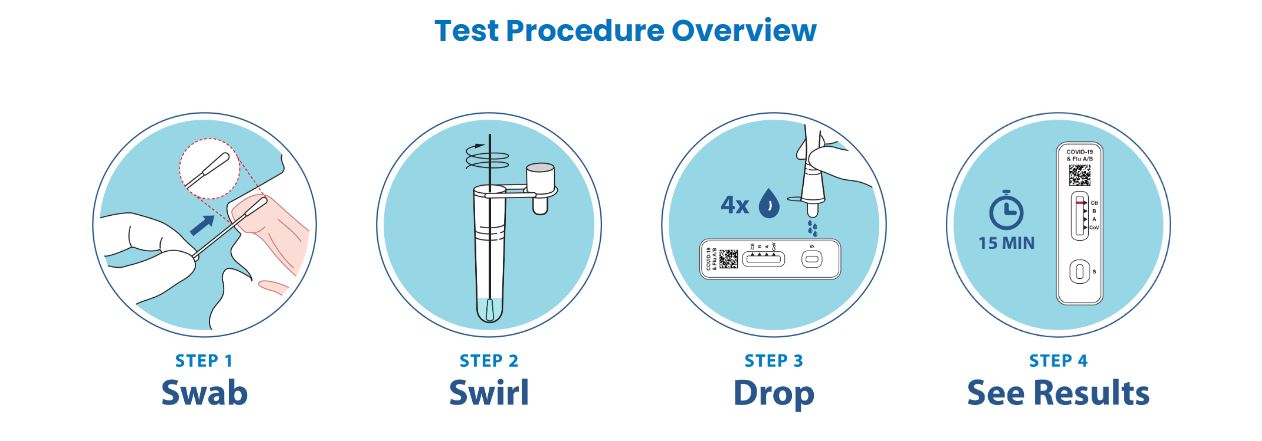
This test procedure overview does not replace the Quick Reference Instructions (QRI). Before you begin the test, it is important to read and follow the detailed instructions in the QRI.