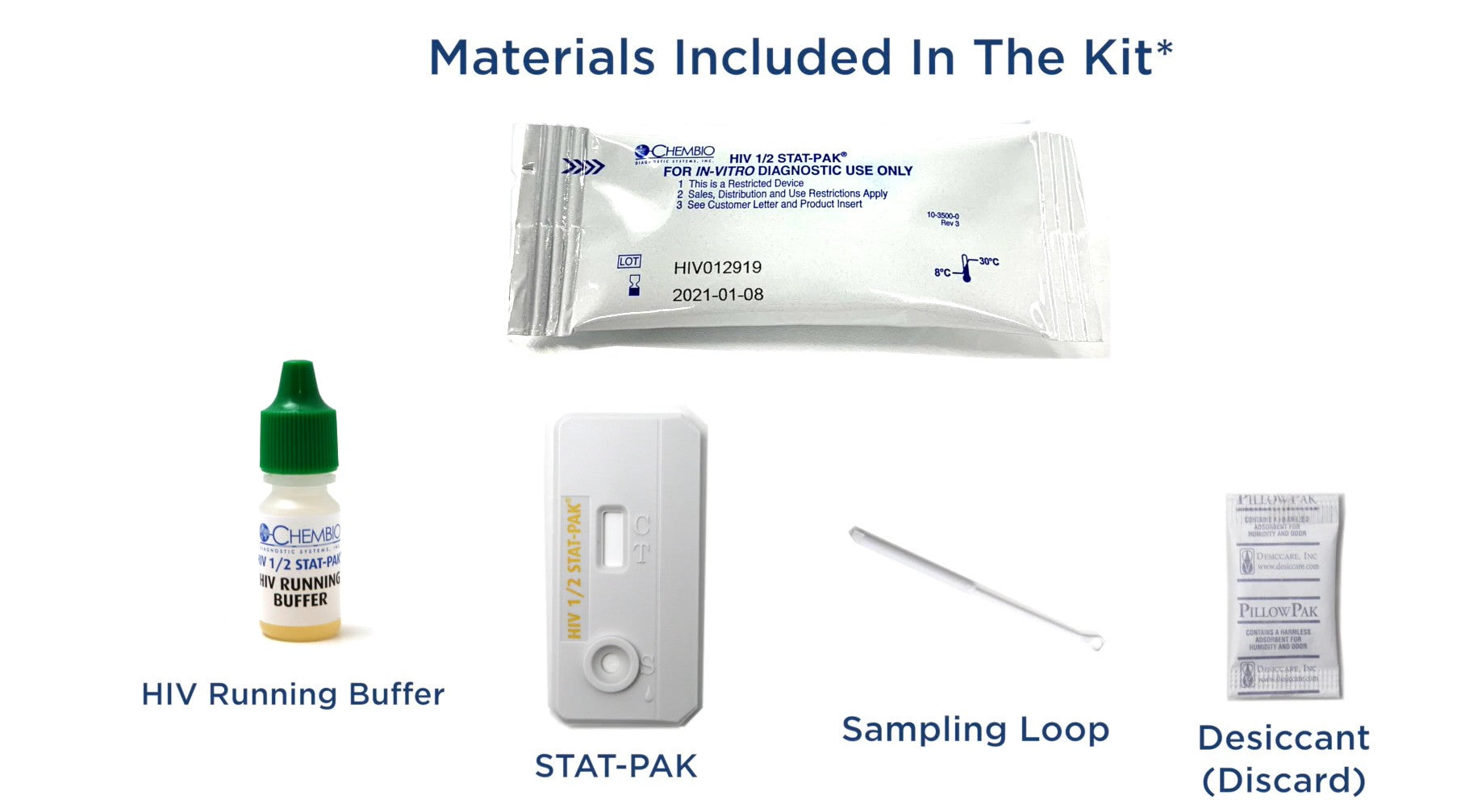
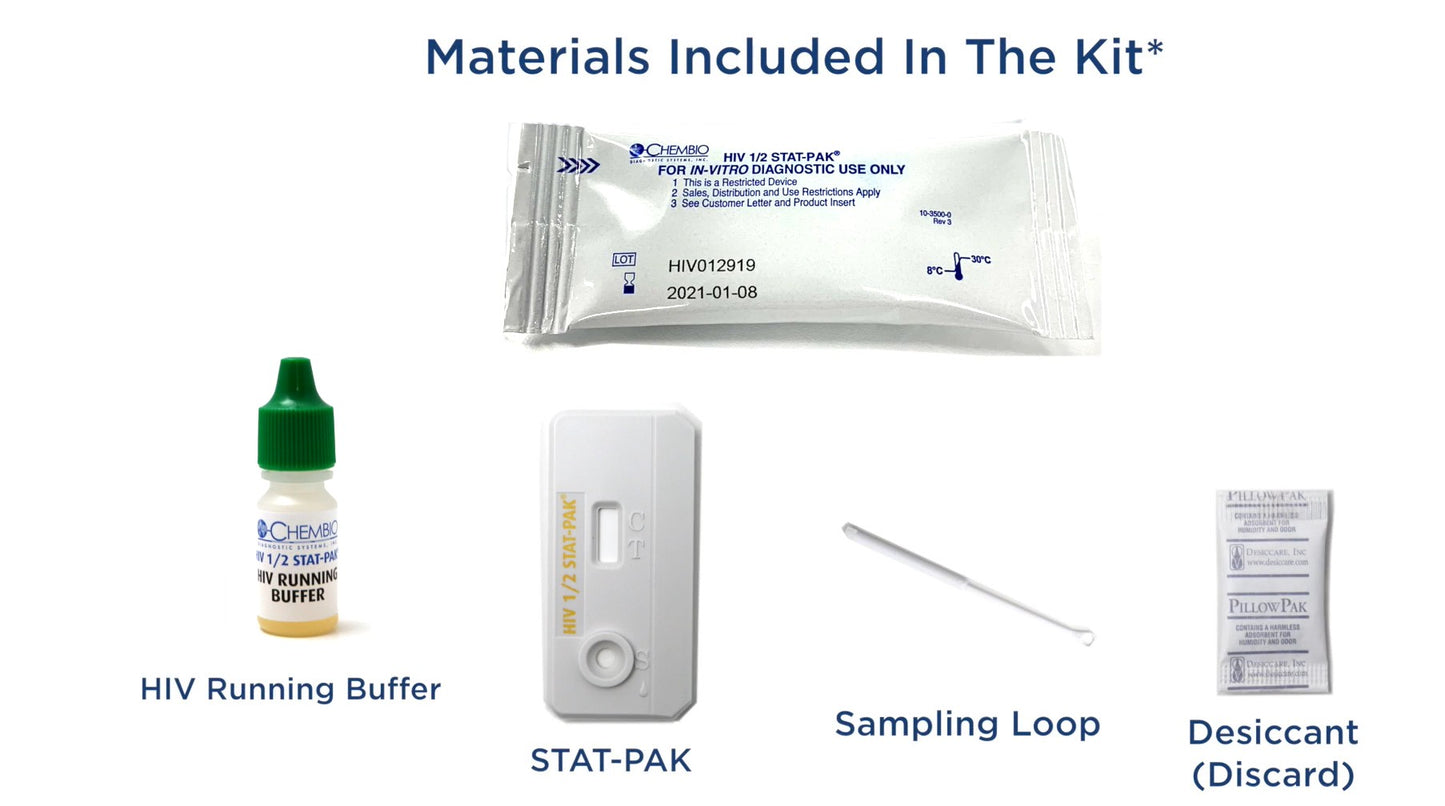
HIV 1 / 2 STAT-PAK Assay, 20 tests/kit (CDI 60-9505-1)
An FDA approved, rapid point-of-care assay for the detection of HIV-1 and HIV-2 antibodies in fingerstick whole blood, venous whole blood, serum or plasma.
Superior Performance
Sensitivity: 99.7%; Specificity: 99.9%1
Innovative Design
Small sample volume: 5 μl
Built-in procedural control
Easy to Use
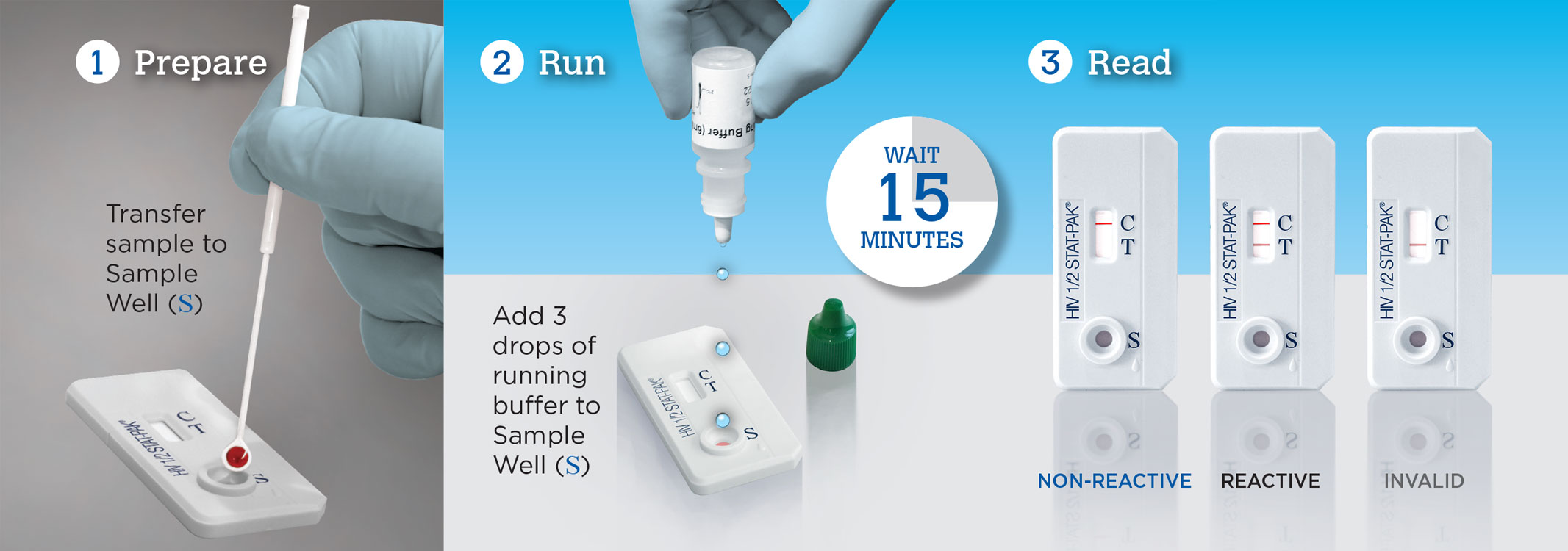
Test in 3 easy steps: Prepare, Run, Read
Requires minimal training
Convenient, Reliable and Cost Effective
- Ideally suited for point-of-care testing
- Rapid reporting: Results in 15 minutes
- 99.7% sensitivity (HIV-1), 99.9% specificity
- Built-in IgG procedural control
- Easy to perform
- No special preparation of reagents required
- Room temperature storage 8° to 30°C (46° to 86°F)
- Long shelf life
- No special equipment required
- Each control pack contains sufficient volume to run 50 tests (available separately)
Test for HIV in 3 Easy Steps with STAT-PAK
Product Information
| Information Type | Product Detail | |
| Sample Volume | 5 μl | |
| Method | Lateral Flow | |
| Test and External Controls Shelf Life* | 24 months | |
| Storage Conditions | 8 - 30°C (46 - 86°F) | |
| Time to Results | 15 minutes | |
| CPT Codes | 86703 or 86703QW, G0435 (for Medicare) | |
Ordering Information
| Product | Catalog Number | |
| HIV 1/2 STAT-PAK® Assay FDA Approved, CLIA Waived | 60-9505-1 | |
| HIV Rapid Test Control Pack | 60-9549-0 | |