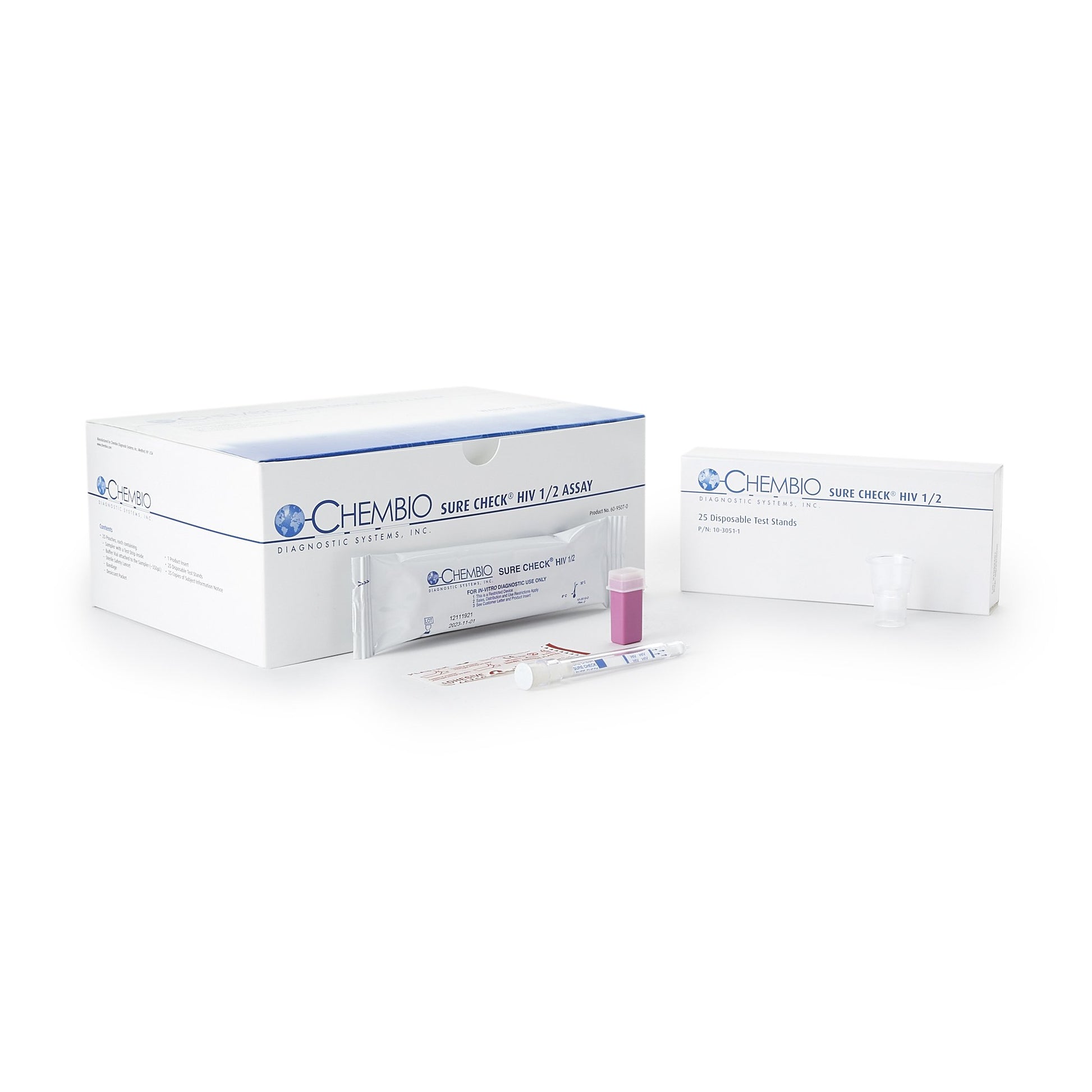
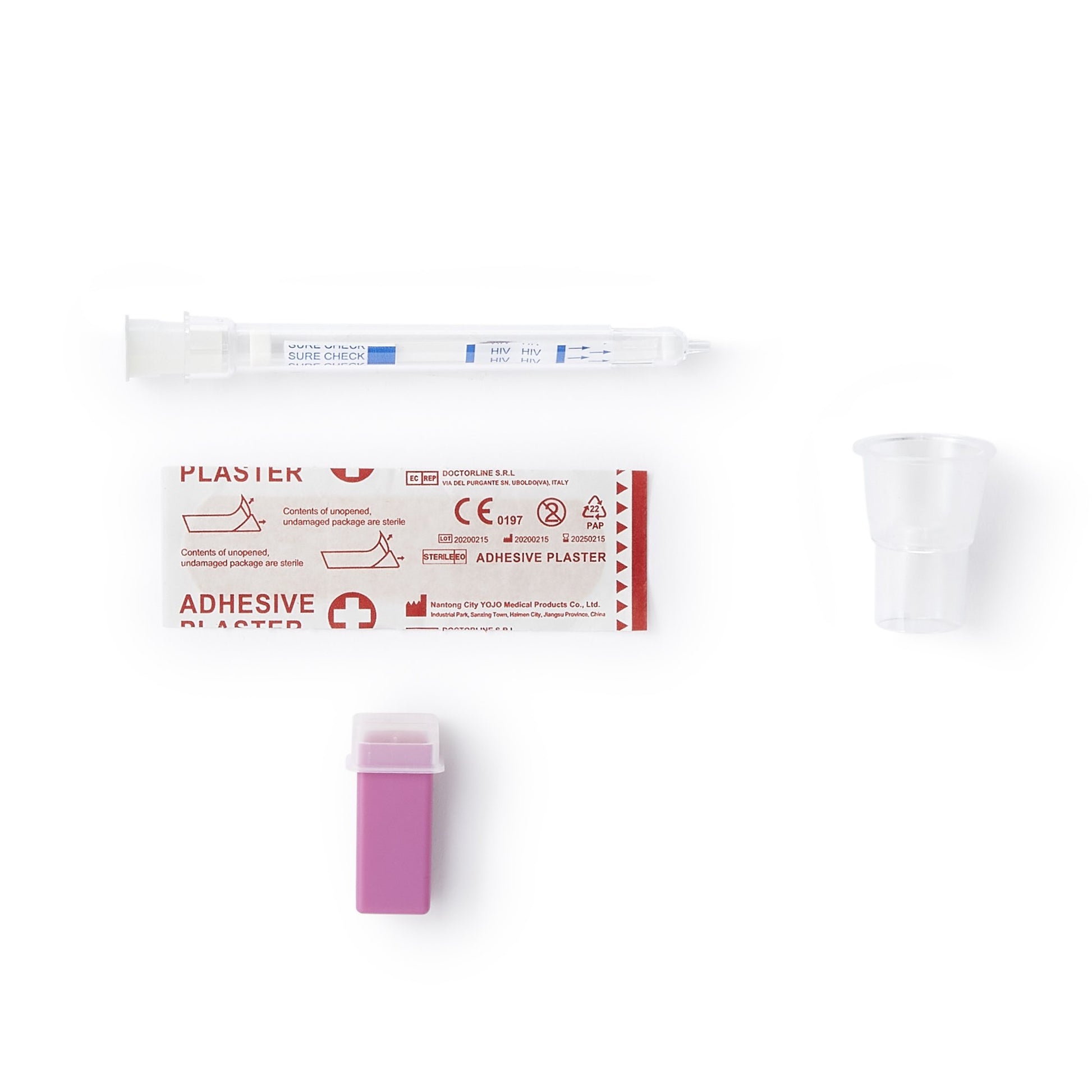
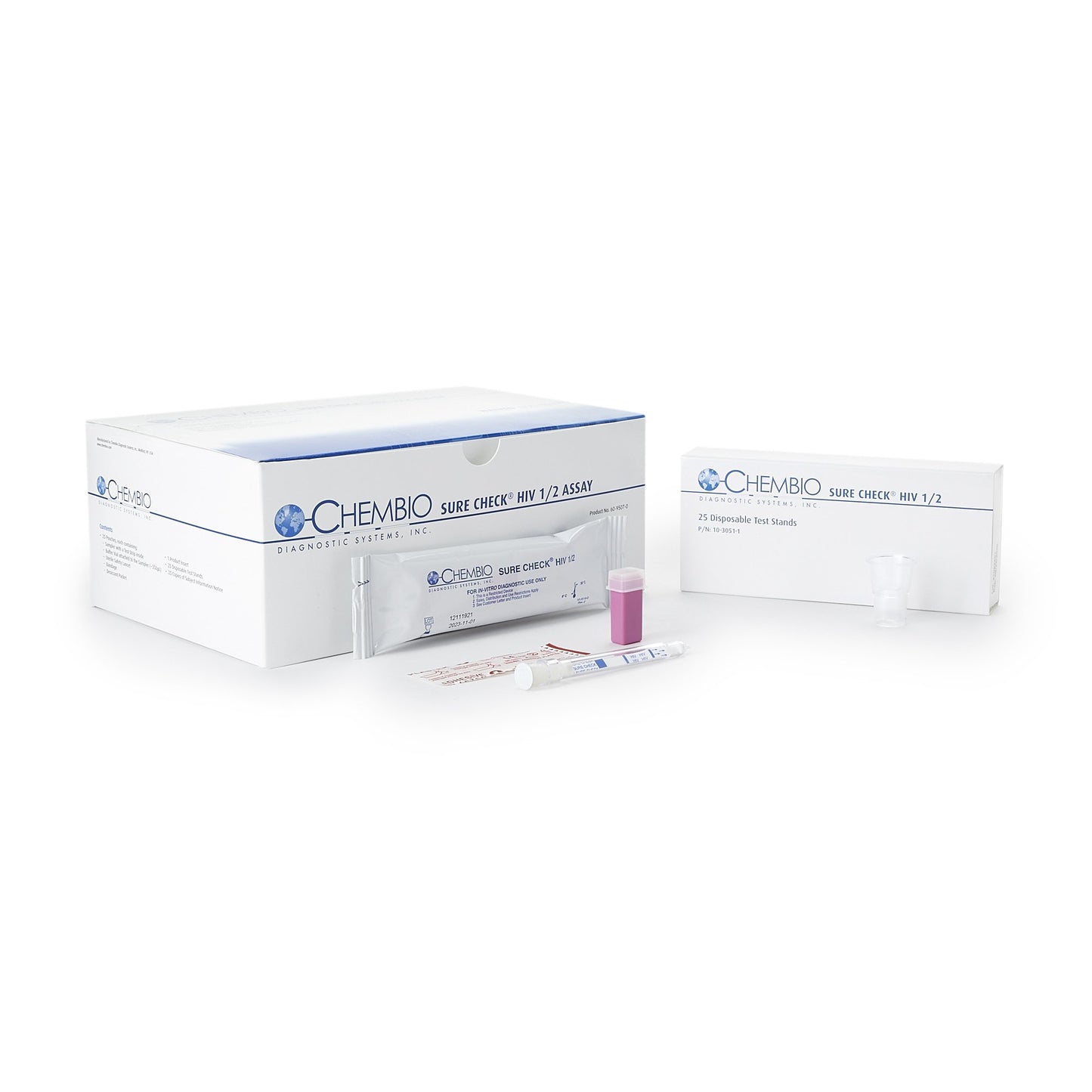
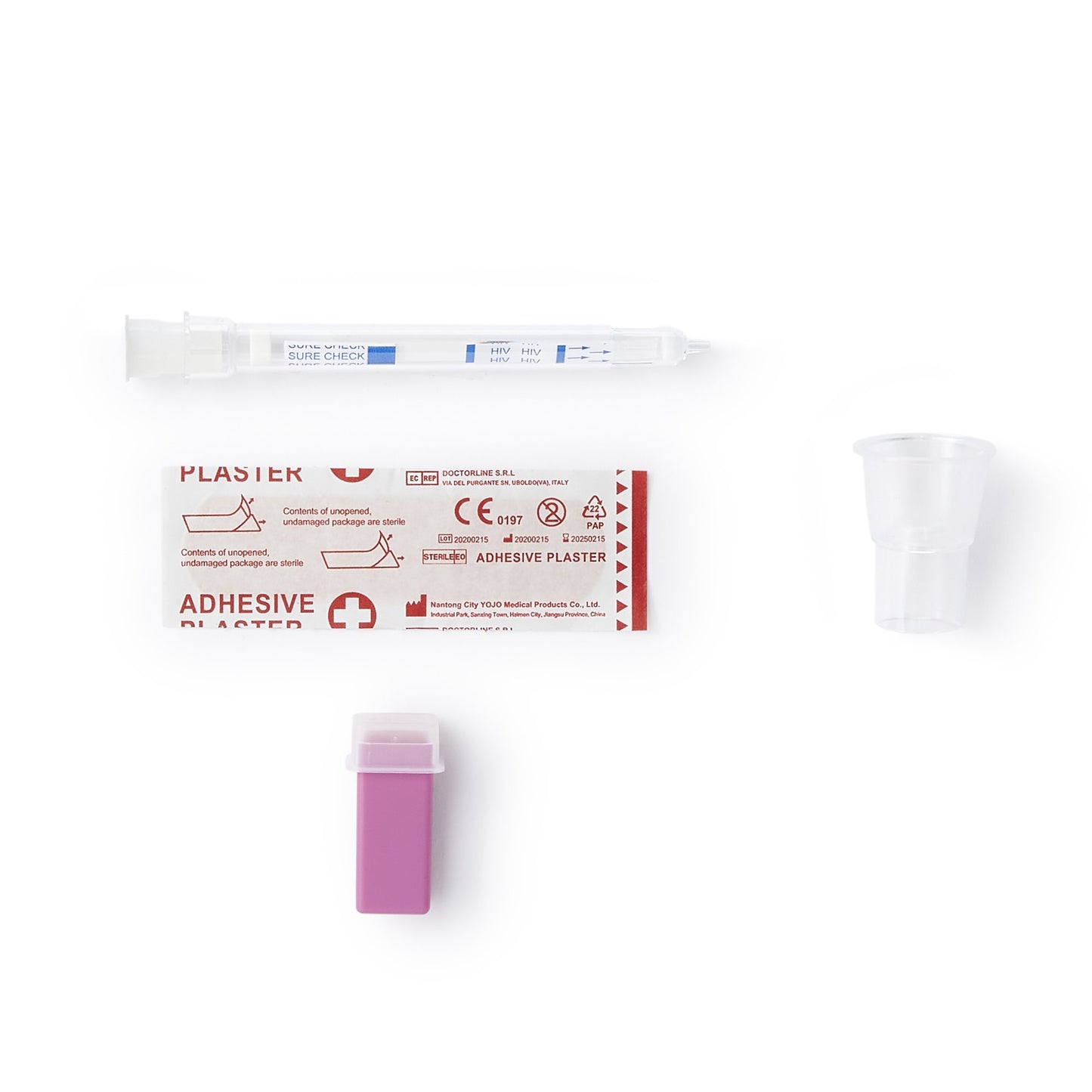
SURE CHECK HIV 1 / 2 Assay, 25 tests/kit (CDI 60-9507-0)
An FDA approved CLIA Waived rapid point-of-care test that detects antibodies to HIV-1 and HIV-2 in fingerstick whole blood, venous whole blood, serum and plasma specimens.
Innovative
- Unique Barrel Design: All-in-one sample collection and test device
- World’s smallest sample size: 2.5 μl
Convenient
- A self-contained unit with minimal hands-on-time
- CLIA waived for fingerstick and venous whole blood
- Contains safety lancet and bandage
Reliable
- Uses GP120, GP41, & GP36 HIV Biomarkers for enhanced antibody detection
- High Accuracy: Sensitivity = 99.7%; Specificity = 99.9%*
- Built-in procedural control
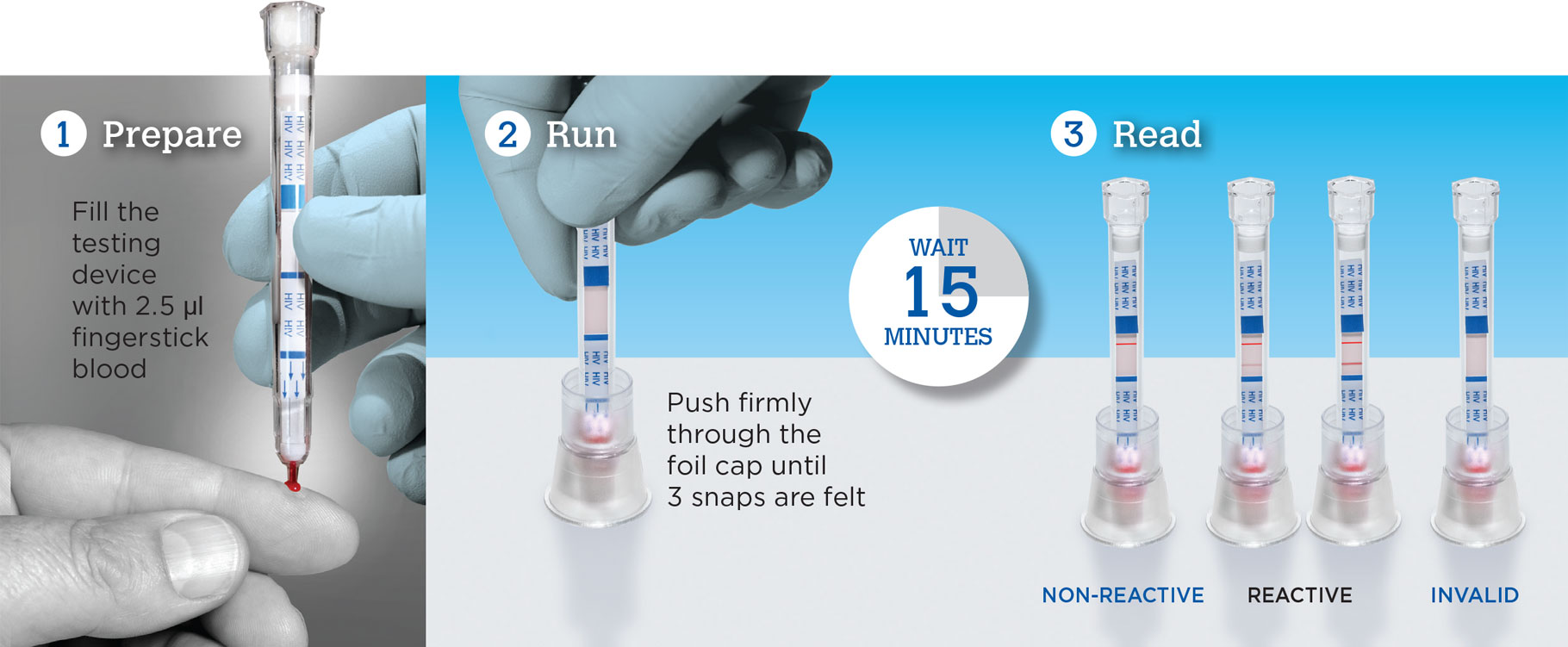
Test for HIV in 3 Easy Steps with SURE CHECK
| Manufacturer # | 60-9507-0 |
| Brand | Sure Check |
| Manufacturer | Chembio Diagnostic |
| Country of Origin | USA |
| Application | Sexual Health Test Kit |
| CLIA Classification | CLIA Waived for Whole Blood / CLIA Moderate Complexity for Serum, Plasma |
| Contents | (25) Test Devices (25) Disposable Test Stands (25) Subject Information Notices (1) Quick Reference (for CLIA Waived) (1) Product Insert |
| Number of Tests | 25 Tests |
| Reading Type | Visual Read |
| Sample Type | Whole Blood / Serum / Plasma Sample |
| Specialty | Immunoassay |
| Test Format | Test Device Format |
| Test Kit Type | Rapid Test |
| Test Name | HIV-1/2 |
| Test Type | Antibody Test |
| Time to Results | 15 Minute Results |
Product Information
| Information Type | Product Detail | |
| Sensitivity | 99.7% | |
| Specificity | 99.9% | |
| Method | Lateral Flow | |
| Time to Results | 15 minutes | |
| Test and External Controls Shelf Life** | 24 months | |
| Storage Conditions | 8 - 30°C (46-86°F) | |
| Product | Catalog Number | |
| SURE CHECK HIV 1/2 Assay (25 Tests) FDA Approved, CLIA Waived | 60-9507-0 | |
| HIV Rapid Test Control Pack | 60-9549-0 | |